The Thompson Center hosted its Spring Research Symposium at the NextGen Precision Health Building in March. Speakers were Drs. Sofia Lizarraga (Brown University) and Evdokia Anagnostou (University of Toronto). A special presentation honored Dr. Judy Miles for her many years of dedication to autism research and programming in Missouri.
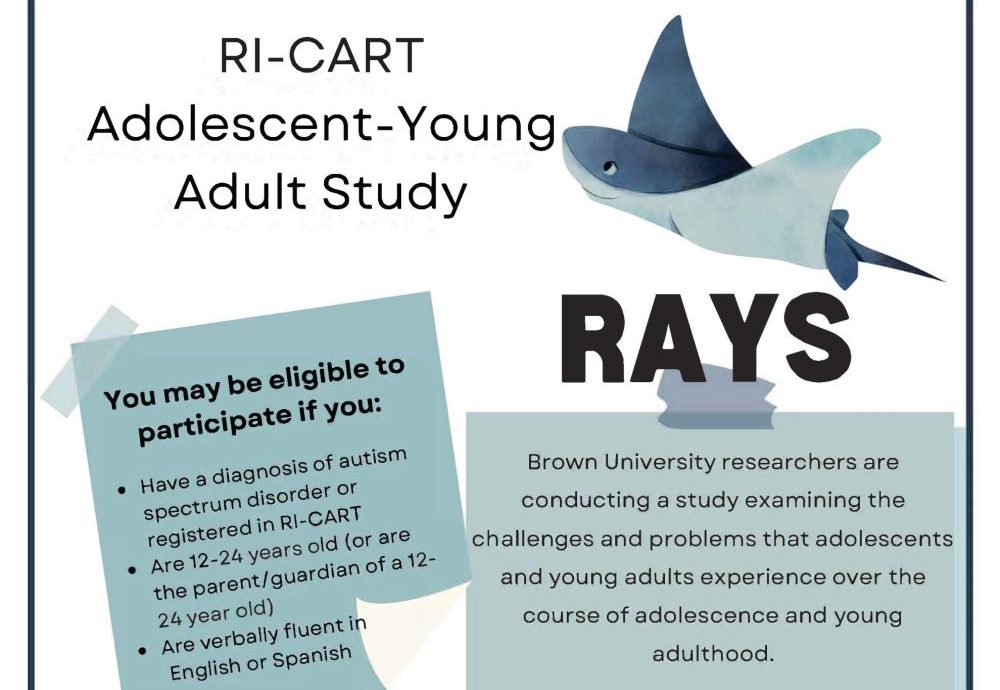
RAYS: Examining the Experiences of Autistic Adolescents & Young Adults
Individuals often begin to drink alcohol during adolescence and early adulthood. But how does this phase of life unfold for autistic teens and young adults compared with their neurotypical peers?
One study at the Thompson Center for Autism & Neurodevelopment at the University of Missouri is focused on investigating the coming of age of autistic teens, particularly in terms of substance use, mental health, and socialization.
This study is an outgrowth of the Rhode Island Consortium for Autism Research and Treatment (RI-CART), which was funded by the Simons Foundation Autism Research Initiative (SFARI) and led by Dr. Stephen Sheinkopf, prior to his role as executive director of the Thompson Center. The RI-CART Adolescent-Young Adult Study (RAYS) is now a collaborative effort between the Thompson Center and Brown University and is funded by the National Institutes of Health (NIH). RAYS launched in 2021 and is jointly directed by Dr. Sheinkopf, Dr. Christina Jackson from Brown University’s Center for Alcohol and Addiction Studies, and Dr. Anthony Spirito, an expert in adolescent mental health at Brown University’s Department of Psychiatry and Human Behavior.
RAYS is following individuals ages 12-24 years using an accelerated longitudinal design. To do so, each participant is studied for a three-year period. Then, advanced research methods are used to compile the data and derive a pattern of what development trends look like across the full age range. RAYS is conducted 100% online using annual surveys that ask questions about the participant’s social environment, mental health, and alcohol and drug use.
The information collected in this study will help researchers begin to understand ways that alcohol use and experiences in autistic young adulthood are similar to or different from that of their typically developing peers, an area that has been the subject of very few studies in the past. Further analysis and data collection could identify additional variables that may be indicators of risk for drug or alcohol abuse or mental health problems, specifically for young adults with autism.
More than 120 autistic teens and young adults have participated in the RAYS project so far, with more than 30% enrolling through the Thompson Center. RAYS will be accepting new study participants through 2024. Eligible adolescents and young adults with a professional autism diagnosis can earn up to $395 for completing the study and their parent or caregiver can earn up to $225. Learn more about getting involved in this study and others at thompsoncenter.missouri.edu/autism-research/join-a-study/.
UM Board of Curators Approves New Thompson Center Project
A proposal to build a new facility for the Thompson Center for Autism and Neurodevelopment was approved by the University of Missouri Board of Curators at its meeting on February 9. The roughly 74,000 square foot building will allow us to serve even more people through our clinical services, research, and training programs. The Thompson Center expects to move into the new facility in December 2025.
Learn more in the press release from the Board of Curators here.

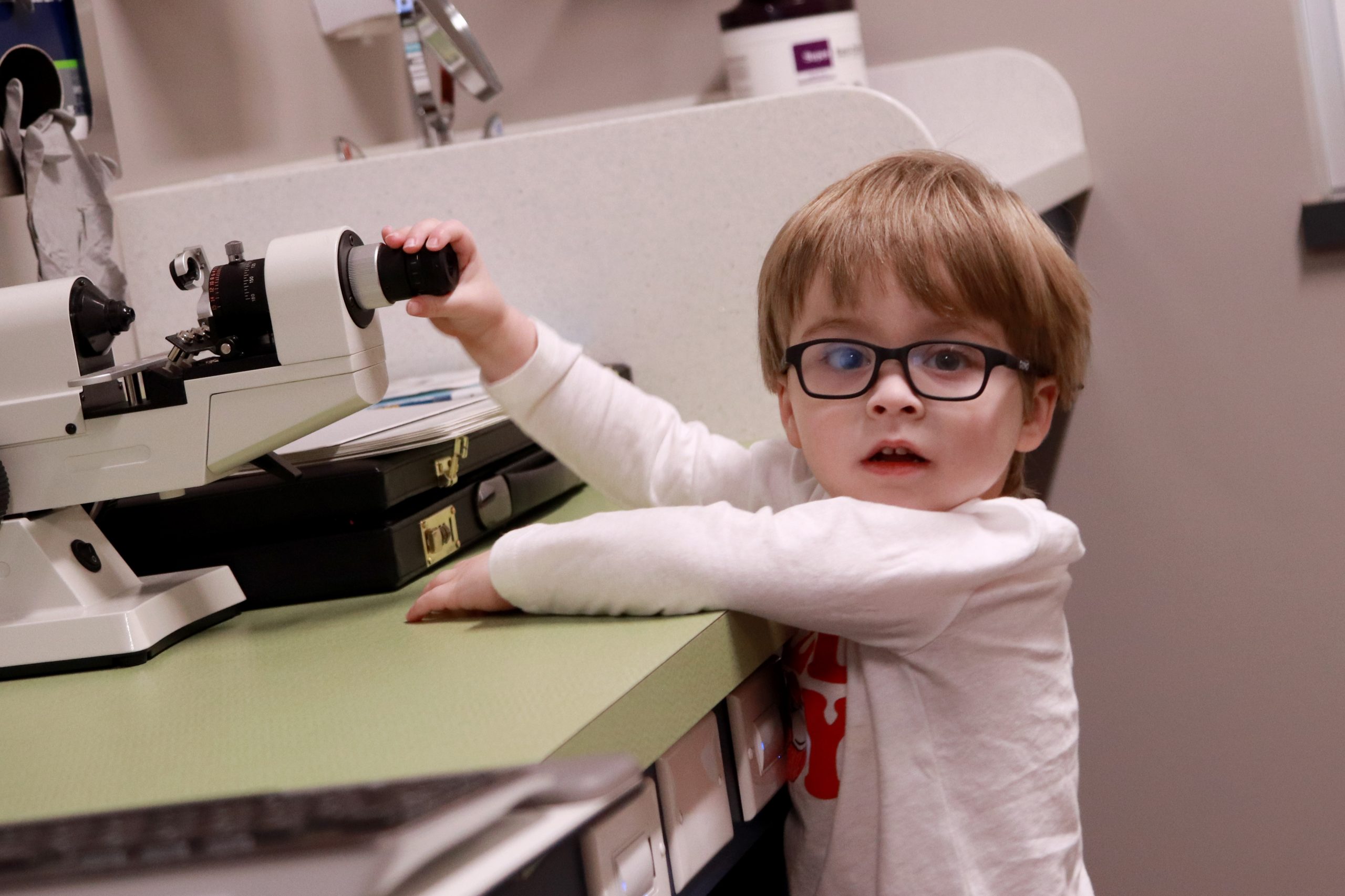
Thompson Center & Mason Eye Institute Partner for Autism-Friendly Vision Screenings
The Thompson Center for Autism and Neurodevelopment partnered with the Mason Eye Institute to provide vision screenings to seven children with autism. Prescription glasses were also provided at no cost to those that needed them. This event was made possible by through sponsorship from the Healthy Vision Association.
Eye exams can be particularly challenging for autistic children. The unfamiliar procedures and deviation from routine can be distressing. The sensory experience of the exam itself is unpleasant for many people, but particularly so for those with sensitivities. Providers at the Mason Eye Institute have received training through the Thompson Center’s Autism Friendly Business program which prepared them with best practices for welcoming and supporting patients with autism.
Keep an eye out for information about more events like this in the coming months!




Winners of Student Poster Session Announced
The Thompson Center for Autism and Neurodevelopment hosted its Student Poster Session at the 17th Annual Thompson Center Autism Conference in October. The session had the most poster submissions to date as well as record-breaking attendance. There were a variety of posters showcasing the breadth of neurodevelopmental research in the fields of psychology, special education, physical therapy, social work, occupational therapy, applied behavior analysis, and educational, school, and counseling psychology. Congratulations to the following winners of this session:
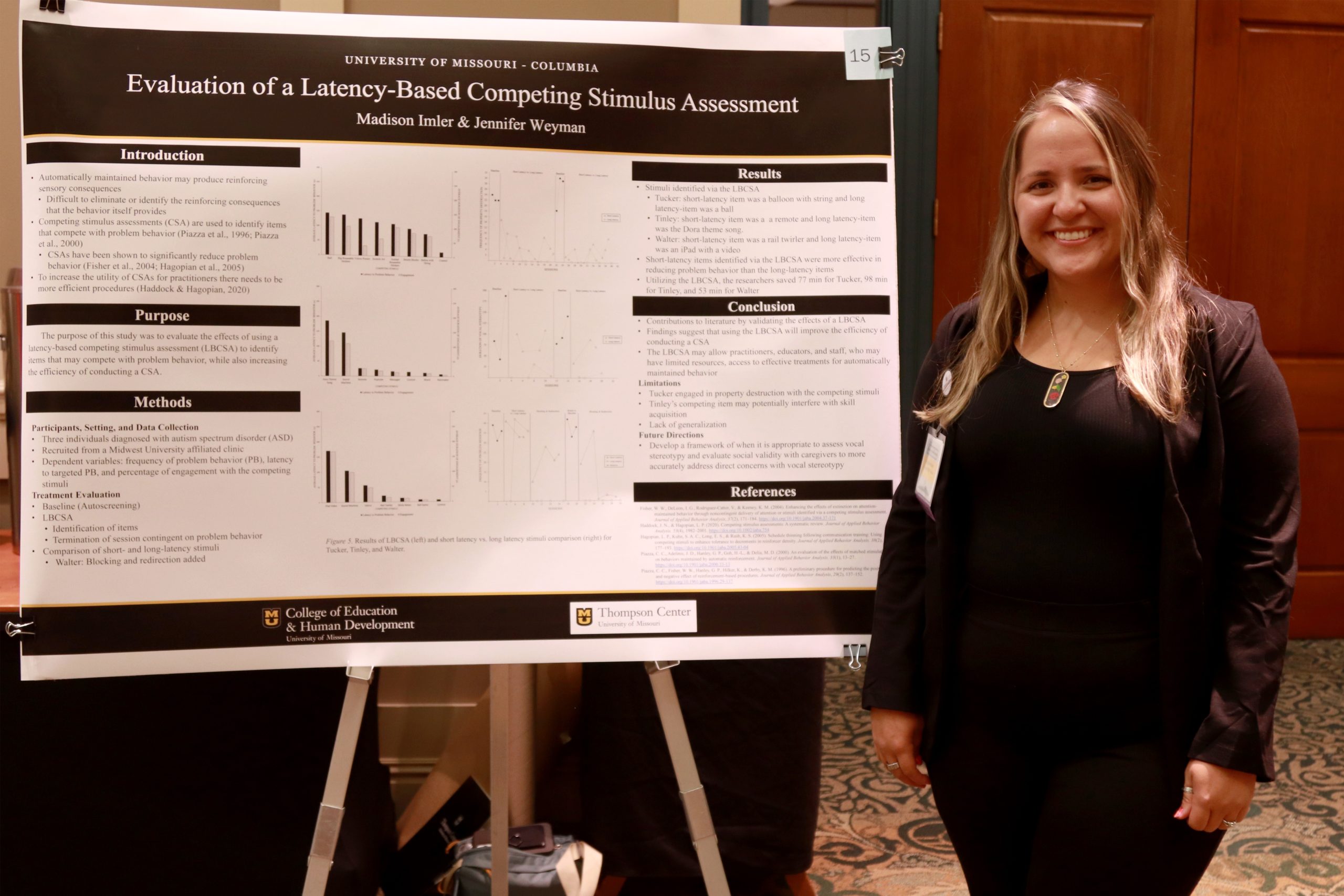
1st Place: Evaluation Of A Latency-Based Competing Stimulus Assessment (LBCSA) by Madison Imler – MU Special Education ABA

A competing stimulus assessment (CSA) is used in the treatment of automatically maintained problem behavior to identify items that compete with the sensory consequences that are associated with the targeted problem behavior. This study aimed to evaluate a more efficient means of conducting a CSA by evaluating the effectiveness of a latency-based competing stimulus assessment (LBCSA). During the LBCSA, a therapist presented potential competing stimuli to the participants, and contingent on the occurrence of problem behavior the session was terminated. The primary researcher recruited three participants that attend a Midwest university-affiliated applied behavioral intervention clinic. To be included in this study, the individual had an ASD diagnosis and was referred to the study by the participant’s Board Certified Behavior Analyst (BCBA). The participants included Tucker who was a 12-year-old male that engaged in property destruction in the form of ripping, picking off, or crushing items. Tinley was a 6-year-old female that engaged in vocal stereotypy in the form of noncontextual vocalizations. Walter was a 10-year-old male who engaged in property destruction by throwing items. All sessions took place within individual rooms at the clinical facility. If the targeted problem behavior was property destruction, the session room was baited with items selected by consulting with the participant’s current clinical team. The primary dependent variables that were measured in this study were the frequency of problem behavior, latency to targeted problem behavior, and percentage of engagement with the competing stimuli. Each problem behavior was individually defined as the primary researcher consulted with the participant’s clinical team, and directly observed the targeted behavior before the start of baseline. In this study, the researchers utilized a multielement design to identify competing stimuli with short latency to disruptive behavior and competing stimuli with long latency to disruptive behavior. In addition, researchers evaluated the comparison of short-latency and long-latency items by utilizing a multielement design embedded within a reversal (ABAB) design. This study contributes to the literature by providing practitioners, educators, and staff with additional resources to further develop function-based interventions. These efficient and effective procedures also allow for behavior analysts to have a more practical and time-efficient assessment to train service providers (Luiselli et al., 2020). Overall, the use of the LBCSA increased the efficiency of the CSA and identified effective competing stimuli for the three participants that engaged in automatically maintained problem behavior. These findings suggest that using the LBCSA will improve the efficiency of conducting a CSA and make the application of the assessment more feasible for practitioners, educators, and staff members. As a result, the LBCSA will positively impact the development of effective treatments for automatically maintained problem behavior.
2nd Place: Feasibility of Transcutaneous Vagal Nerve Stimulation in Youth with ASD by Roee Dar – MU School of Medicine

The purpose of this pilot study is to evaluate the feasibility and tolerability of auricular transcutaneous vagal nerve stimulation (tVNS) as a non-pharmacological, non-invasive anxiolytic therapy in children and teens with autism spectrum disorder (ASD). Feasibility and tolerability were assessed by treatment compliance and participant feedback. Youth with ASD frequently suffer from anxiety as a comorbid condition that can be pharmacologically resistant (Kirsch et al., 2020). tVNS offers a minimal-risk method of shifting autonomic nervous system activity away from the sympathetic overactivation that contributes to anxiety (Lamb et al., 2017). tVNS has been studied in adult and pediatric populations with various neuropsychiatric conditions (Yap et al., 2020) but not as treatment for anxiety in youth with ASD. Methods & Results This is an open-label feasibility trial conducted from October 2021–September 2022. Participants with diagnosed ASD aged 7-17 were identified for recruitment through the Thompson Center Database or flyers at the Thompson Center Clinic. Baseline participant anxiety was assessed using the Revised Childhood Anxiety Sensitivity Index (CASI-R), Parent Rated Anxiety Scale-ASD (PRAS-ASD), and both a clinician- and a caregiver-version of the Clinical Global Impression of Severity. Only participants scoring moderate or high anxiety on the CASI-R were eligible for participation. Participants were then calibrated, fitted, and trained along with their caregivers to use the Soterix© mini-CT Stimulator transcutaneous electrical nerve stimulation device. Caregivers were instructed to administer a pre-programmed 60-minute period of electrode stimulation to their child nightly for two weeks. Compliance was measured with the device log and defined as use of the device on at least 80% of the nights during a participant’s trial. Caregivers were given an administration and behavior log to maintain throughout the study. At the two-week follow-up visit, all baseline assessments were repeated in addition to the clinician- and caregiver-versions of the Clinical Global Impression – Improvement Scale (CGI-I), and the caregiver log was returned. Of the fifteen enrolled participants, ten were compliant and included in data analysis of anxiety measures. Excluded participants included two that failed the baseline anxiety requirement, one that withdrew consent pre-treatment, and two that were non-compliant. Mean compliance of the twelve participants that received the device was 87%. The device was well tolerated by participants, with comments including “best sleep I ever had” and “it feels good”. Reasons for non-compliance included technical difficulties and failure to incorporate the device into a nightly routine. Among compliant participants, average anxiety measures on the CASI-R decreased by 7.9 points (SD = 6.3) and on the PRAS-ASD by 15.1 points (SD = 11.9). The median impression by clinicians and caregivers on the CGI-C was “minimally improved” anxiety. Implications for the Field tVNS treatment in youth with ASD is feasible and tolerable with potential benefits for anxiety. The results of this pilot study will inform larger, randomized control trials assessing the efficacy of tVNS as anxiolytic treatment. tVNS can potentially offer a simple, minimal-risk therapy that can reduce the pharmacological burden on youth with ASD while improving their independence and quality of life.
3rd Place: Intraindividual Variability in Subjective Sleep and Average Fatigue in Parents of Children on the Autism Spectrum by Braden Hayse – MU Clinical Psychology

Intraindividual Variability in Subjective Sleep and Average Fatigue in Parents of Children on the Autism Spectrum Background: Fatigue is related to various adverse health outcomes. Mean levels of some common sleep variables, such as total sleep time (TST), sleep onset latency (SOL), and wake after sleep onset (WASO), have been associated with fatigue. However, intraindividual variability (IIV) of sleep parameters might play an independent role in sleep’s relationship with fatigue. Understanding fatigue is particularly important for parents of children with autism spectrum disorder (ASD) given fatigue’s negative associations with positive parenting and implementation of child interventions. Objective: The objective of this preliminary study was to examine linear associations between IIV of subjective sleep parameters and mean fatigue levels in parents of children on the autism spectrum. Methods: The sample included 66 parents who expressed interest in a behavioral treatment sleep study for their school-aged children diagnosed with ASD (6-12 years old; NCT04545606). All parents (Mage=37.03, SD=6.53; 91% female) completed daily electronic diaries over a two-week baseline period. Daily fatigue rating was collected using a visual analog scale (0-100) and averaged within individuals. Within-individual standard deviations of subjective TST, SOL, and WASO were calculated to estimate IIV. Data were analyzed in R (v4.1.2) using multiple linear regression models controlling for participant age, gender, and individual respective sleep parameter means. Results: Bivariate correlations between sleep variable IIV and average fatigue indicated a positive association between TST variability and average fatigue, r(64)=0.33, p<0.01. Multiple regression analyses showed that greater IIV of TST was associated with higher average fatigue (β=0.14, 95%CI [0.01, 0.27], sr2=0.06, p=0.041). No significant associations were found between average fatigue level and IIV of WASO or SOL. Conclusion: Results suggest that greater TST variability may be one factor independently contributing to higher fatigue levels in parents of children on the autism spectrum, which warrants further examination of sleep variability and its associations in this population. Increased insight into the connection between parent fatigue and sleep might inform the importance of considering sleep interventions for both children and parents, and potential subsequent indirect treatment benefits. Future research could explore IIV of additional sleep parameters, fatigue IIV as an outcome, alternative methods of sleep measurement, and study designs that address causation.
Clinical Collaboration: Thompson Center Researchers Partner with MU Health Care Clinics
The summer before David Beversdorf began his fellowship, he attended a workshop where one message rang clear: research should be driven by a question, not a technique. Having a clinic at his fingertips as a researcher at the Thompson Center for Autism and Neurodevelopment is a helpful recruitment asset in many contexts, but was not the right technique for the job when he wanted to study the effects of stress during pregnancy on microRNA biomarkers that could be used as an indicator of autism. “We already know that there is some association between prenatal stress and autism,” said Beversdorf, “but we hope to better understand the link so we can hone in on the risk factor.”
The methodology for this study involves administering surveys about stress to pregnant women and collecting saliva samples from which concentrations of microRNA can be measured. The key to accomplishing this is recruiting pregnant women, a demographic that the Thompson Center clinic does not provide services for.
“So, this research question led us to collaborate with Dr. Goodman’s clinic,” said Beversdorf, “they have the population we don’t have that’s needed to explore this question.” Dr. Jean Goodman is the Griffin Endowed Chair and Professor in the University of Missouri’s Department of Obstetrics, Gynecology, and Women’s Health as well as the director of MU Health Care’s Maternal Fetal Medicine clinic, which sees roughly 1,500 patients annually. This study aims to collect survey data and saliva samples from 150 participants at their second trimester ultrasound appointment during its one-year pilot phase funded by BioNexus, a private academic research partnership in Kansas City.

“We here at the University of Missouri – Columbia are working to improve pregnancy outcomes for our mothers and their babies,” said Dr. Goodman. “Doing so in a multidisciplinary, collaborative way – like this study – allows expertise from varied specialties to come together in a unified way with the common goal of improving maternal-child health.”
The BioNexus study is not the first time Thompson Center researchers have sought partnerships with other clinics for recruitment. The Thompson Center has been testing REACH, a mobile-based screening tool for early identification of autism, since November 2021 and will be concluding trials in October 2022. The goal is to develop a tool that families can use for at-home screening; however, in its current form, the screening tool must be administered by professionals.
This study requires a sample of children from the general population to determine the effectiveness of the app. “If kids are coming to the Thompson Center, someone is already concerned, and they’re more likely to screen positive,” said Dr. Kerri Nowell, the principal investigator on REACH at the Thompson Center. To this end, the team of researchers partnered with MU South Providence Pediatrics, which sees approximately 2,000 children ages 0-3 for well child visits annually.
Research Specialist Julie Muckerman said this partnership created a new question: “What does it look like to do a research visit at a pediatric appointment?” The answer involved collaborating with every pediatrician and resident at the clinic in order to reach the most families with information about the study. Outreach through practitioners will continue to be a key tool as REACH evolves from its current testing phase to a ready-to-use screening tool. “We want to identify high-risk kids without requiring them to come to a specialty clinic,” said Muckerman, “and one of the best ways to reach them is through their pediatrician.”
The Thompson Center will soon be bringing another study into its collaboration with the Maternal Fetal Medicine clinic. The Early Years Study will be looking at characteristics of infant cry as early indicators of autism and other developmental disabilities. This study funded by the National Institute of Mental Health was brought to the Thompson Center by Dr. Stephen Sheinkopf when he became the Executive Director in September 2021.
“If we want to know about development in early infancy, we need to make contact with families early on,” Dr. Sheinkopf said, explaining the need for clinical partnership. “We don’t serve infants at the Thompson Center, yet.”
The Early Years Study is the Missouri site of this NIMH-funded study. The initial location, called the Rhode Island Neurobehavior Observation Study (RhINOS), involves a partnership between Women and Infants Hospital of Rhode Island, Brown University, and the University of Rhode Island. The methodology for RhINOS is based around reaching out to parents and offering the opportunity to enroll while mothers are still in the mother-baby unit after delivery. When adapting the study for recruitment in Missouri, Maternal Fetal Medicine stood out as a centralized location for many expecting parents, so the project adapted so that the Early Years team could approach mothers during their prenatal visits.
“There is a natural synergy between Early Years and Dr. Beversdorf’s work on the BioNexus project,” Dr. Sheinkopf added in his reasoning for working with Maternal Fetal Medicine. Both studies are contingent on recruitment at the earliest stages of development and have a common goal of ultimately gaining insight into early identification of autism and other neurodevelopmental disorders.
Despite their shared recruitment source, the eligibility standards vary between the two studies. Early Years has its sights set on the general population. “We’re looking to recruit a broad group of parents to participate,” said Dr. Sheinkopf. “Most newborns will be eligible for this study.”
The BioNexus study will be recruiting from a narrower subset of patients at Maternal Fetal Medicine: Black and African American women. As a historically marginalized group, these women of color are underrepresented in research. They are also at a higher risk of experiencing stress factors during pregnancy. Dr. Goodman echoed the need for this nuance in research. “The association between mental well-being and pregnancy-related outcomes is particularly evident in our most vulnerable populations, those patients of color.”
“Clinical partnerships go beyond the research relationship,” Dr. Nowell said of the experience she had working with South Providence Pediatrics. Working closely with providers and staff at other practices creates opportunities to discuss when a child should be referred for an autism evaluation and a deeper understanding of the Thompson Center’s processes and services. “We now have advocates there.”
Muckerman explained that recruitment for REACH has already made an impact on the clinic at the Thompson Center. “Many of the participants that screened positive have joined our waitlist,” she said. “They are starting on a path to receive services.”
Researchers On The Go
Dr. Stephen Sheinkopf presented on current trends in autism research as part of the NextGen Precision Health Discovery Series in August. His lecture featured highlights from his experience with studying infant cry, including the Early Years study.

Students in several disciplines presented their research at the annual Student Poster Showcase

Several Thompson Center researchers attended the International Society for Autism Research (INSAR) Annual Meeting in Austin, TX in May, including Dr. David Beversdorf and a group of graduate students. Left to right: Matthew Prendergast, Taeseon Woo, Dr. Beversdorf, Carrina Appling, Candice King, Nanan Nuraini.

MU Scientist Links Epigenetic Biomarkers to Gastrointestinal Issues for Kids with Autism
Findings could have future implications for precision medicine, lead to individualized treatments
As a clinician at the University of Missouri Thompson Center for Autism and Neurodevelopmental Disorders, David Beversdorf helps patients with autism spectrum disorder (ASD), many of whom may also be struggling with gastrointestinal or digestive issues, including constipation and diarrhea. These symptoms are experienced by children with ASD at a higher rate than their neurotypical peers, although some individuals might not respond favorably to traditional treatments, such as laxatives.
In a recent study, Beversdorf collaborated with a researcher at Penn State University to identify specific RNA biomarkers linked with gastrointestinal issues in children with autism. The findings could help one day lead to individualized treatments aimed at easing the pain of these individuals.
Saliva samples were collected from nearly 900 children, some of whom had autism and experienced gastrointestinal disturbances, at several academic medical centers across the country. After analyzing the samples, the researchers identified specific RNA biomarkers linked to children who had autism and experienced gastrointestinal symptoms.
“We wanted to understand how a child’s body responds to the various bacteria living in the mouth and determine if these interactions contribute to gastrointestinal symptoms,” said Steve Hicks, an associate professor of pediatrics at the Penn State College of Medicine, who collaborated with Beversdorf on the study. “By identifying these specific microRNAs in the saliva of children with autism, these molecules may serve as future targets for developing novel treatments or tracking medication effectiveness in children with autism-related gastrointestinal conditions.”
Beversdorf added that RNA have regulatory properties throughout the human body, and the specific RNA identified in the study may have regulatory effects on biological pathways related to metabolism, digestion, depression and addiction.
“It’s one of those ‘chicken or the egg’ cases where we still don’t know if it is the RNA potentially contributing to the gastrointestinal issues, or if the gastrointestinal issues are causing the RNA to be expressed differently, but we have identified a relationship, which will be useful to further explore going forward,” said Beversdorf, who also has appointments in the MU College of Arts and Science and MU School of Medicine. “This research can potentially help contribute to precision medicine one day, where we can follow children with autism and gastrointestinal symptoms over an extended period of time and assess how they might respond to personalized treatments, with the ultimate goal of reducing their symptoms and improving their quality of life.”
“Saliva RNA biomarkers of gastrointestinal dysfunction in children with autism and neurodevelopmental disorders: Potential implications for precision medicine” was recently published in Frontiers in Psychiatry. Funding for the study was provided by the National Institutes of Health. Co-authors on the study include Kristin Sohl, David Levitskiy, Priscilla Tennant, Robin Goin-Kochel, Rebecca Shaffer, Alexandra Confair and Frank Middleton.
Highlighting the promise of personalized health care and the impact of large-scale interdisciplinary collaboration, the NextGen Precision Health initiative is bringing together innovators from across the University of Missouri and the UM System’s three other research universities in pursuit of life-changing precision health advancements. It’s a collaborative effort to leverage the research strengths of Mizzou toward a better future for Missouri’s health. An important part of the initiative is its anchoring facility, the Roy Blunt NextGen Precision Health building, opened in October 2021, which expands collaboration between researchers, clinicians and industry leaders in a state-of-the-art research facility.
Story Contributor:
Brian Consiglio, MU News Bureau
The Early Years Study: A New Look Into Infant Cry
The Early Years Study is the latest project to begin the recruitment phase at the Thompson Center for Autism and Neurodevelopment. This project will utilize audio recordings of infant cries in search of a new way to identify early signs for autism and developmental disabilities.
Researchers have been studying infant cry since the 1950s, but Dr. Stephen Sheinkopf is among the first to focus on it as a predictor of autism. Dr. Sheinkopf, who joined the Thompson Center as its Executive Director in 2021, began studying atypical vocalizations in young children with autism in the late 1990s and released his first paper on the subject in 2000. Following that, he began to use infant cry to study vocalizations in young infants. A paper published in 2012 led to funding from the National Institutes of Health (NIH) to develop the analytical algorithm used for the current iteration of Dr. Sheinkopf’s infant cry research.

The Rhode Island Neurobehavior Observation Study (RhINOS) was funded by the National Institutes of Mental Health (NIMH) in late 2019 and with the goal of following children in a longitudinal study starting in March 2020. Dr. Sheinkopf, the principal investigator, was at Brown University and Women and Infants Hospital in Rhode Island at the time. He partnered with researchers at the University of Rhode Island on the project.
The original methodology involved recording cries in a clinical setting; however, the onset of the COVID-19 pandemic forced the research team to redesign the study so parents could submit recordings using a smartphone. While allowing for remote recording posed some technical challenges, the data collection process became more convenient for participants, thus creating the opportunity to gather even more audio samples for the study.
After completing a postpartum survey with demographic information and family history, parents are asked to create recordings of their infant’s cry when they are 2-6 weeks old. The recordings are submitted to the research team via a mobile app for analysis. The team then uses specialized software to measure a large number of features for each cry sample, some of which are indistinguishable to the human ear.
Researchers ultimately hope their analysis will show patterns in the cries that can be used to identify infants with a high risk of autism. “We want to know how we can translate our findings into care,” said Dr. Sheinkopf. The sooner autism is diagnosed, the faster children and their families can be connected with therapies and support services.
The RhINOS and Early Years studies plan to follow 2,700 children from birth to age three. Nine hundred mothers of infants and pregnant women have been recruited in the first year.
Research Spotlight: Dr. Stephen Sheinkopf
Dr. Stephen Sheinkopf joined the Thompson Center as Executive Director and Thompson Endowed Chair in Child Health in September 2021.
What brought you to the Thompson Center?
I came to the Thompson Center last September for the opportunity to lead an integrated team focused on the population that I have spent by career caring for and about.
What are your research interests?
My research is focused on autism as well as neurodevelopment more broadly. A lot of my work has been about studying the differences in development and outcomes amongst children with autism and those without autism. Currently I lead the Early Years Study here at Mizzou that is testing ways to identify likelihood for autism beginning in infancy. But I am also interested in the experiences of people with autism across development. We have additional research that is following adolescents into early adulthood.
What is something you hope to better understand by the end of your career?
There are two major things I want to explore. First, I want to understand how we can predict autism from the earliest stages of development so we can provide supports as early as possible. Intellectually, I am fascinated by the way autism presents differently as children age. I want to know more about why signs of autism are often so subtle in infants but grow to be more pronounced in toddlerhood and beyond.
What’s the best thing about working in this field?
The type of relationships I get to develop with my patients and their families is unique in this population. With the nature of my research and clinical practice, I often work with the same patients for many years through the course of their development. It is truly gratifying to create these ongoing connections.
What study have you been most excited about?
I love infancy. The Early Years study allows me to bring together my two passions of studying infants and studying autism.
Now that you have been with the Thompson Center for a year, what is your vision for moving forward?
After immersing myself in the Thompson Center’s work firsthand, I have an even deeper commitment to making our center a model for team-based care that brings together research, training, and clinical service to have the greatest impact for our patients and their families.